NEW TO THE STREET TV Features Advancements in Leading
Technologies on the Fox Business Network- MARCH 25, 2018 at
1:30 PM EST/ 10:30 AM PST
Broadcasting Nationwide, FOX Business Network Brings Viewing
Access to 95 million homes
NEW YORK, March
23, 2018 (GLOBE NEWSWIRE) -- “NEW TO THE STREET” brings to
the viewing audience a spectrum of companies, each on the
threshold of breakthrough in their respective markets.
NEW TO THE
STREET TV airs nationwide on the FOX Business Network on
Sunday, March 25, 2018 at 1:30 PM Eastern/10:30 AM Pacific–
reaching 95 million homes. Check your local cable provider’s
channel lineup to find Fox
Business Network in your area. Featured participants
include:
- Dthera
Sciences (OTCQB:DTHR) The Company’s CEO Ed
Cox, who recently co-chaired the Digital Therapeutics
and Digital Summit. An event designed to inspire future
leaders in this new field of medicine, aimed to define
the current and future landscape of this rapidly
emerging industry. The event drew over 100 digital
medicine executives, pharmaceutical leaders, and venture
capitalists. Mr. Cox will be summarizing the results of
that event and updating shareholders on the Company’s
vision for the future.
-
Solar Integrated Roofing Corporation (OTCPINK:SIRC) Following
an extremely pleasing record breaking quarter, Mr. David
Massey presents how the team continues to grow, as
demand strengthens. Advantageous deals were recently
reached with both Angie’s List and Home Advisor, giving
the Company a distinct advantage in their strategy of
building a national footprint. With the Company’s
completion of its first eight installations for Lowe’s,
Massey will provide insight on management’s vision
moving forward.
-
PetVivo Holdings, Inc. (OTCQB:PETV)
Mr. John Lei will be interviewed for details regarding
the Company’s highly anticipated U.S. Patent and
Trademark Office (USPTO) approval for patents granted on
two of the Company’s signature products. As the Company
advances toward commercial production, Mr. Lei will be
presenting PetVivo’s strategy to successfully drive the
Company’s revolutionary patented products into the
marketplace, thus generating meaningful revenues.
-
Reign Sapphire Corp. (OTCQB:RGNP)
Chief Executive Officer, Joseph Segelman will be
updating investors on advancements toward the launch of
the Company’s highly anticipated Initial Coin Offering
(“ICO”). As the Company approaches the release of “Reign
Coin’s” white paper, Mr. Segelman to keep its
shareholders and others fully informed on the Company’s
latest developments.
Ken Evseroff, a
“NEW TO THE STREET” Television Anchor, states, “We have come
to know these Companies and the cutting-edge nature of their
technologies fairly well. And, we are very pleased to be
showcasing each individually on the Fox Business Network’s
‘NEW TO THE STREET’ TV business Show. With our focus on
growth emerging companies, we have discovered the pivotal
role that timing plays in the successful execution of
opportunity. We empower investors with “Due Diligence” and
research well prepared and presented. ‘NEW TO THE STEET’
knows that being in the right place at the right time is
vital when investing in this market.”
FMW Media Works
Corp.’s “NEW
TO THE STREET” is a leading provider of
business profiles and special corporate televised
programming. FMW Media Works produces “NEW TO THE STREET”
which paves the way to the latest financial issues, offering
a blend of business and financial services news reporting
and in-depth interviews relating to new products, economic
analysis, and public company profiles. Airing as paid TV
programing, the show’s potential reach of viewer reaches 100
million homes in the USA, and potentially 5.3 million
viewers in Canada. Other international stations also air the
program. To learn more visit the NEW TO THE STREET’s
website- www.newtothestreet.com.
Forward-Looking
Statements Disclaimer:
This press release contains forward-looking statements
within the meaning of Section 27A of the Securities Act of
1933, as amended, and Section 21E of the Securities Exchange
Act of 1934, as amended. In some cases, you can identify
forward-looking statements by the following words:
"anticipate," "believe," "continue," "could," "estimate,"
"expect," "intend," "may," "ongoing," "plan," "potential,"
"predict," "project," "should," "will," "would," or the
negative of these terms or other comparable terminology,
although not all forward-looking statements contain these
words. Forward-looking statements are not a guarantee of
future performance or results, and will not necessarily be
accurate indications of the times at, or by, which such
performance or results will be achie
Dthera Sciences Commends the Work of the Alzheimer's Association
to Raise Awareness of the High Cost of the Disease on Healthcare
System and Caregivers
Study Reports that Alzheimer's Dementia Affects 5.7 Million
Americans Dthera Developing First Digital Therapeutics to Address
Issues Associated with Dementia
SAN
DIEGO, March 23,
2018 /PRNewswire/ -- Dthera Sciences
(OTCQB:DTHR), a digital therapeutics company developing
quality-of-life therapies to address dementia from
Alzheimer's disease and other neurodegenerative conditions,
today commended the Alzheimer's Association report released
earlier this week for highlighting the serious state of the
disease, and particularly the high cost it exacts on the
healthcare system and the patient caregivers. The "2018
Alzheimer's Disease Facts and Figures" report stated that
the overall costs to care for the estimated 5.7 million
Americans with Alzheimer's disease and other dementias will
reach a total of more than $277
billion in 2018 for the second year in a
row. The cost is expected to increase significantly, as the
number of Americans over the age of 65 increases from 53
million today, to 88 million by 2050.
The report also points out the
strain on caregivers, with one study citing that 48% of all
caregivers for the elderly are caring for patients with some
form of dementia. The report also highlighted the long
duration and intensity of this type of care. Fifty seven
percent of familial caregivers, according to another study
cited in the report, have provided care for more than four
years. The average time a caregiver spends caring for a
patient with dementia is 92 hours per month, which is 27
more hours per month than care for patients without
dementia. Dementia-patient caregivers also experience higher
levels of emotional stress and financial burden than those
who care for other types of patients. Poignantly, the report
goes on to say that the "intimacy, shared experiences, and
memories that are often part of the relationship between a
caregiver and care recipient may also be threatened...[as
the dementia advances]."
"This important report from the
Alzheimer's Association points out the devastating effect of
Alzheimer's dementia on the US healthcare system, especially
the toll it takes on caregivers, both familial and
professional," said Edward
Cox, Dthera Sciences CEO. "Dthera is focused on
creating and delivering digital therapeutics that bring
medically-validated treatments, such as reminiscence
therapy, to patients suffering from dementia and severe
forms of social isolation, to ease symptoms and create a
better quality-of-life for them and their caregivers. Dthera
expects to launch its first digital therapeutic product,
ReminX, a tablet that delivers personalized reminiscence
therapy, in the second quarter or 2018. We hope that ReminX
will have a meaningful impact on this terrible disease, that
has few viable treatment options."
About Digital Therapeutics
Digital therapeutics, a new and
emerging subsection of digital health, is a technology that
delivers a therapy directly to a patient via a digital
interface. The sector offers the promise of scaling
effective individual therapies to larger patient
populations, thereby amplifying care, changing patient
behavior, and reducing the cost-of-care.
About Dthera Sciences
Dthera Sciences, based in San
Diego, is a publicly-traded digital therapeutics
company developing and commercializing innovative
quality-of-life therapies addressing dementia, and other
social isolation issues, associated with the elderly and
neurodegenerative conditions, such as Alzheimer's disease.
The company's lead product, ReminX, digitally delivers a
clinically-supported therapy, reminiscence therapy (RT), to
patients suffering from dementia, and extreme social
isolation, to reduce anxiety and improve quality-of-life. An
open-label study in patients with dementia, conducted at the University
of California San Diego, showed a significant
reduction in anxiety, depression and overall emotional
distress after the patients viewed their story over ReminX.
The company plans to launch ReminX commercially in the
second quarter of 2018.
For more information, please
visit www.dthera.com and www.reminx.com
SAN DIEGO, Feb. 27, 2018 /PRNewswire/ -- Dthera Sciences (OTCQB:DTHR),
a digital therapeutics company focused on developing innovative
digital quality of life therapies for neurodegenerative diseases, is
pleased to announce its CEO, Edward Cox, has been selected to
co-chair the Digital Therapeutics and Digital Medicine Summit (DTxDM)
alongside David Benshoof Klein, CEO of Click Therapeutics, a company
developing software as prescription medical treatments.
Click Therapeutics CEO, David Benshoof Klein, commented, "As
co-chair of this inaugural summit, I am delighted that over 100
digital medicine executives, pharmaceutical leaders, and venture
capitalists are coming together to share, collaborate, and advance
validated digital interventions."
"I am honored to co-chair this event alongside David, especially as
it is the first event of its kind and is the beginning of
introducing Digital Therapeutics to the broader healthcare
community," said Dthera Sciences CEO, Edward Cox. "I think we may
look back at this event as a very unique gathering of people that
will go on to be the leaders in this new field of medicine."
Taking place on February 27 [th] -28 [th] , this summit is aimed to
define the current and future landscape of this rapidly emerging
industry.
https://digitaltxsummit.com/
The event is the first ever gathering of leaders in this
fast-growing industry and, in addition to Mr. Cox and Mr. Klein, a
number of other notable speakers will be present including:
MORE
CurePSP & Dthera Sciences Announce Initial Positive Results of Pilot
Program
SAN DIEGO, Oct.
4, 2017 /PRNewswire/
-- Dthera Sciences (OTCQB:DTHR), a digital therapeutics company
focused on developing innovative digital quality of life
therapies for neurodegenerative diseases and oncology, announced
today the positive initial results of a pilot Program with
CurePSP, a leading nonprofit focused on neurodegenerative
diseases.
CurePSP and Dthera recently engaged in a
pilot program in which individuals diagnosed with PSP used
ReminX, Dthera's leading digital therapeutic designed to reduce
anxiety and improve quality of life. The initial results for PSP
were decidedly positive and thus, CurePSP and Dthera have agreed
to work together to make the ReminX product available for the
wider CurePSP community with the hope of gathering additional
information as well as making a positive impact in the quality
of life in as many PSP patients and their families as possible.
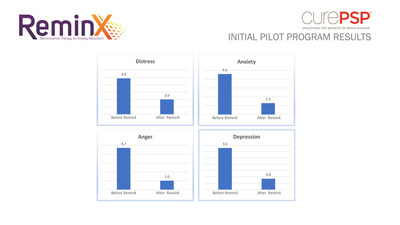
The pilot program tested several factors
to determine the effectiveness of ReminX. Caregivers asked PSP
patients how they felt before and after viewing ReminX,
including a score from 1-10 indicating how distressed, anxious,
depressed and angry patients felt. On average, there was a
reduction of all symptoms. The product was used in an acute
setting and results in a chronic setting are not yet known.
"I am very encouraged with the initial
findings from the pilot program and believe that through working
together with CurePSP, we can make ReminX available to more
members of the PSP community and continue to gather critical
data on how to make the most significant positive impact for
those suffering from neurodegenerative diseases," said Dthera
Sciences CEO, Edward Cox.
David Kemp, President of CurePSP, added,
"We are delighted to be working with Dthera Sciences on this
exciting project, as it offers the promise of improving the
lives of patients and families suffering from PSP and related
diseases. While these diseases are fatal and largely
untreatable, ReminX holds the promise of greatly improving
quality of life while we search for a cure."
About CurePSP
CurePSP is the leading nonprofit advocacy
organization focused on prime of life neurodegenerative diseases
– a spectrum of fatal brain disorders that often strike during a
person's most productive and rewarding years. Currently there is
no treatment or cure for these diseases, which affect more than
150,000 people in the U.S. alone. Since it was founded in 1990,
CurePSP has funded more than 180 research studies and is the
leading source of information and support for patients and their
families, other caregivers, researchers and doctors and allied
healthcare professionals. CurePSP is based in New
York City. Please visit www.curepsp.org for
more information.
About Dthera Sciences
Dthera Sciences, based in San
Diego, CA, is a digital therapeutics company focused on
developing innovative digital 'quality of life' therapies for
neurodegenerative diseases and oncology. The Company's lead
product, ReminX, is an artificial-intelligence-powered digital
therapeutic designed to reduce anxiety and improve quality of
life in patients with Alzheimer's disease and Dementia. For more
information, please visit www.dthera.com and www.reminx.com
About Digital Therapeutics
Digital Therapeutics is a new subsection
of digital health that strives to directly deliver a therapy via
use or interaction with software technology. The goal of Digital
Therapeutics is to mirror an effective treatment and use
technology to scale it to a larger patient population, thereby
amplifying doctors' and nurses' care, changing patient behavior,
and most importantly, reducing cost of care.
Forward Looking Statement
This press release contains
"forward-looking statements" as that term is defined in the
Private Securities Litigation Reform Act of 1995, regarding the
research, development and commercialization of therapeutic
products and technologies, as well as the Company's efforts to
increase its customer base and initiate additional clinical
trials. Such forward-looking statements are based on current
expectations and involve inherent risks and uncertainties,
including factors that could delay, divert or change any of the
statements made, and could cause actual outcomes and results to
differ materially from current expectations. No forward-looking
statement can be guaranteed. These forward-looking statements
are made as of the date of this press release, and the Company
expressly disclaims any intention or obligation to update the
forward-looking statements, or to update the reasons why actual
results could differ from those projected in the forward-looking
statements. Readers are urged to read the risk factors set forth
in the Company's most recent annual report on Form 10-K,
subsequent quarterly reports filed on Form 10-Q, and other
filings made with the SEC. Copies of these reports are available
from the SEC's website at www.sec.gov or
without charge from the Company.
About HealthCareReporter.net :
HealthCareReporter.net is
a subsidiary
of Target Publishing Inc, and is a leading publisher of todays
market and investment news, commentary, proprietary research and
videos from seasoned journalists, analysts and contributors
covering the financial markets and global economies We have been
paid in cash by a third party. We do not take shXares
for our work . Leveraging our extensive distribution network and
social media presence, we have cultivated
a valuable audience of
engaged market enthusiasts, which in turn delivers a
variety of unique opportunities for industry partnerships,
corporate communications, market exposure and investment. We
are paid in cash by these companies PBIO - MBVX. We do not take
shares nor own shares in ay of these companies
A complete disclaimer can be viewed HERE
|